Explain the Stability of Different Conformation of N Butane
Conformational analysis is the study of the energetics between different rotamers and is useful for understanding the stability of different isomers by taking into account the spatial orientation and through-space interactions of substituents. The butane molecule is drawn in Chem 3D and energy minimized.
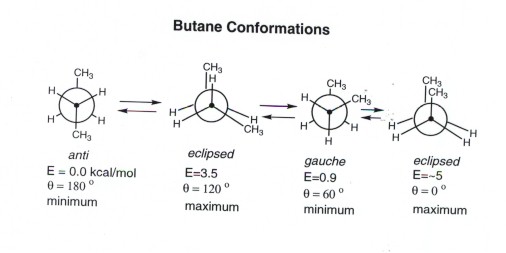
Butane Conformational Analysis
In anti staggered n-butane the methyl groups are placed at a dihedral angle of 1 8 0 0 and the steric hindrance is minimal in anti-form than in gauche form.
. The lowest-energy arrangement called the antiperiplanar or anti conformation is the one in which the two large methyl groups are as far apart as possibleAs rotation around the C2C3 bond. The most stable conformation of butane is the one in which the two terminal methyl groups are the farthest removed from each other ie. To explain further two substituents X and Y on adjacent atoms A and B are in the closest proximity indicating that the torsion angle XABY is 0.
It is the most unstable conformation because the two groups are present at a dihedral angle of 0 which increases the steric strain in the compound. The higher energy of eclipsed bonds is known as eclipsing strain. This is the highest energy conformation for butane due to.
Generally Butane has four conformation isomers which are fully eclipsed gauche eclipsed and anti butane conformational isomers. Heres an energy diagram showing the different conformations we saw in the video. The order of stability is staggered gauche eclipsed energy order eclipsed gauche staggered.
Stereochemistry of Butane. A plot of potential energy against rotation about the C2C3 bond in butane is shown below. Draw Newman and sawhorse projections for the eclipsed and staggered conformations of ethane.
At a dihedral angle of 60 degrees one hydrogen of each of the. Now let us consider butane a slightly larger molecule. The energy profile is symmetrical with a mirror plane at the dihedral angle of 180.
The anti form is the absolute energy minimum since the gauche form has a small steric interaction between the two methyl groups. If different substituents are present all will be chiral. And these pictures are just stills from the actual video.
When the dihedral angle 60 or 300 gauche conformation in butane the methyl groups are farther apart and therefore the potential energy drops by 41 kcalmol. Types The two main classes of isomers are called structural isomers and stereoisomers. Trans isomer has a twofold rotational axis hence it is also achiral.
The conformational possibilities increase as alkanes become larger. This is visualized at 180 dergrees on the graph and is the most stable conformation. There are two energy minima the gauche and anti forms which are both staggered and thus have no torsional strain.
Topic- conformational analysis of n- BUTANE Made by- Sophia Mubashir 1. Arrange the following conformations of n-butane according to their increasing stability. The molecule will normally occupy a steady state one of low energy and experience a change to another steady state only upon absorbing enough energy to reach and pass through the uneven intervening conformation.
Isomerism Isomers are non-identical compounds with the same molecular formula. Both butane and isobutane are gaseous hydrocarbon compounds. In butane the gauche-conformer is less stable than the anti-conformer by about 09 kcalmol.
Actually there are only three the cis-1 3-dimethylcyclohexane has a plane of symmetry and is achiral. When we look at the chemical structure of butane we can see that it has two. Conformations of n-butane are as under Staggered conformation has minimum repulsion so it is the most stable.
This places the molecule at its optimal energy which corresponds to the staggered conformation where the two methyl groups are furthest from each other. Eclipsed conformation occurs in a conformation when hydrogen atoms are attached to two carbons areas nearest to each other as possible. The third conformation is anti conformation which is the most stable of all as the heavier methyl groups are situated opposite each other at a dihedral angle 180.
Isobutane is a structural isomer of butane. Structural constitutional isomers Stereoisomers 3. Butane is an organic compound.
The anti staggered conformation of n-butane is more stable than gauche staggered and eclipsed conformations of n-butane. Staggered conformations about carbon-carbon single bonds are more stable have a lower potential energy than the corresponding eclipsed conformations. When the dihedral angle 0 or 360 syn conformation in butane the two methyl groups are in close proximity with the molecule therefore the potential energy is at its highest.
We started with the staggered conformation of butane right here which has a certain potential energy and we went from this staggered conformation to this eclipsed conformation right here by rotating 60. Lets us discuss these isomers below. They are hydrocarbons because these compounds are composed only of C and H atoms.
Conformational analysis can be used to predict and explain product s. There are now three rotating carbon-carbon bonds to consider but we will focus on the middle bond between C 2 and C 3Below are two representations of butane in a conformation which puts the two CH 3 groups C 1 and C 4 in the eclipsed position. Both butane and isobutane have the same chemical.
CONFORMATION OF BUTANEN-Butane is a four carbon alkane derived from ethane in which one hydrogen atom on each carbon is substituted by a methyl group. 1 3-dimethylcyclo hexane has two chiral centers and can have four stereoiso mers 2 2 4. Butane Conformational Energy Diagram.
Normally when we rotate the molecule of butane at the axis of the C-C bond it shows different conformation isomerism. Gauche-staggered I fully eclipsed IIeclipsed III and -staggered IV. This molecule can be found in several forms known as isomers.
If the forces are strong different conformations vary greatly in energy or stability. Somewhat less favorable is the gauche conformation in which the methyl groups assume a dihedral angle of 60.
Conformational Isomerism In Ethane N Butane And Cyclohexane Pharmaceutical Guidelines

Most Stable Conformation Of N Butane Is Youtube

The Order Of Stability For The Conformations Of N Butane Among These Is Anti I Gauche Youtube
No comments for "Explain the Stability of Different Conformation of N Butane"
Post a Comment